ai architecture
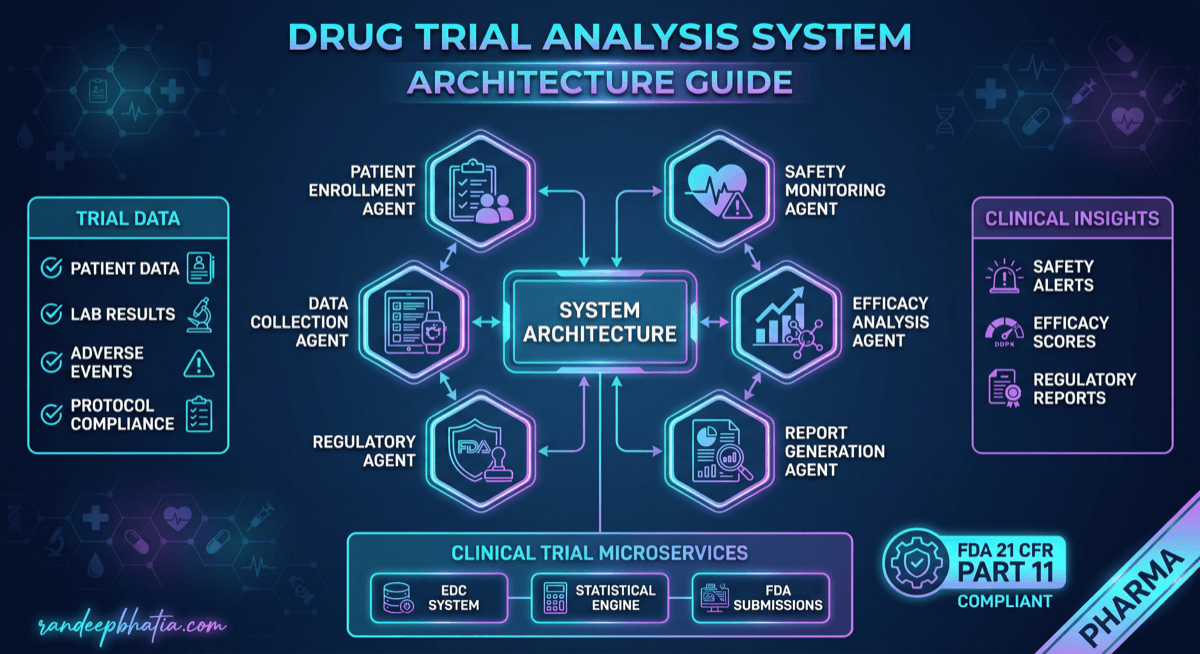
Drug Trial Analysis System Architecture
Production-ready system architecture for drug trial analysis. Includes component design, data flow patterns, scaling strategies, and security considerations.
2025-08-28
View Full Size
Production-ready system architecture for drug trial analysis. Includes component design, data flow patterns, scaling strategies, and security considerations.
2025-08-28
